InVivoMAb anti-mouse Ly6C
Product Details
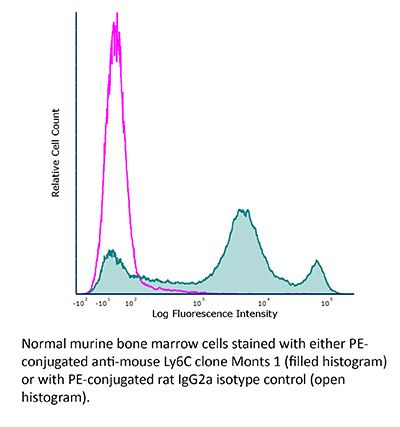
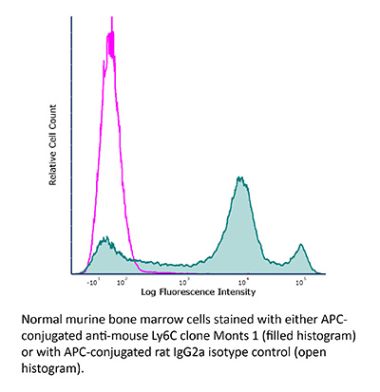
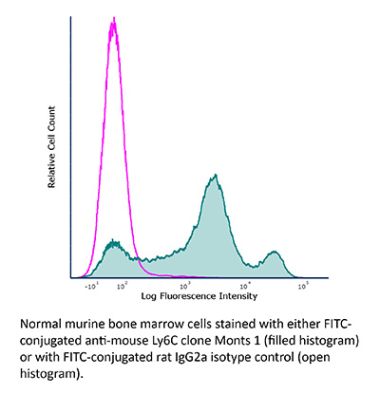
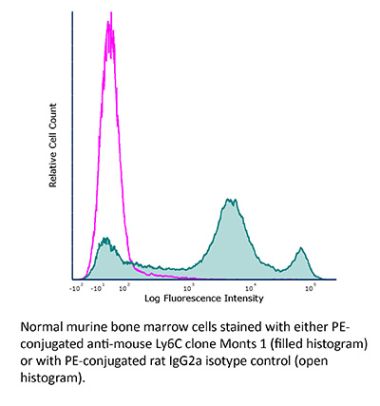
The Monts 1 monoclonal antibody reacts with mouse Ly6C, a 14-17 kDa member of the Ly-6 superfamily of GPI-anchored cell surface proteins. Ly6C is expressed by monocytes, endothelial cells, granulocytes, and some T cell subsets.Specifications
| Isotype | Rat IgG2a |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Not available or unknown |
| Reported Applications |
in vivo macrophage depletion (in combination with clodronate liposomes) Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687696 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vivo macrophage depletion (in combination with clodronate liposomes)
Rowe, A. M., et al. (2017). "Subclinical Herpes Simplex Virus Type 1 Infections Provide Site-Specific Resistance to an Unrelated Pathogen" J Immunol. doi : 10.4049/jimmunol.1601310. PubMed
HSV-1 infections of the cornea range in severity from minor transient discomfort to the blinding disease herpes stromal keratitis, yet most patients experience a single episode of epithelial keratitis followed by re-establishment of a clear cornea. We asked whether a single transient episode of HSV-1 epithelial keratitis causes long-term changes in the corneal microenvironment that influence immune responses to subsequent corneal infection or trauma. We showed that C57BL/6 mouse corneas infected with HSV-1 KOS, which induces transient herpes epithelial keratitis without herpes stromal keratitis sequelae, possessed a significant leukocytic infiltrate composed primarily of CD4+ T cells and macrophages along with elevated chemokines and cytokines that persisted without loss of corneal clarity (subclinical inflammation). Chemokine and cytokine expression was CD4+ T cell dependent, in that their production was significantly reduced by systemic CD4+ T cell depletion starting before infection, although short-term (3-d) local CD4+ T cell depletion postinfection did not influence chemokine levels in cornea. Corneas with subclinical inflammation developed significantly greater trauma-induced inflammation when they were recipients of syngeneic corneal transplants but also exhibited significantly increased resistance to infections by unrelated pathogens, such as pseudorabies virus. The resistance to pseudorabies virus was CD4+ T cell dependent, because it was eliminated by local CD4+ T cell depletion from the cornea. We conclude that transient HSV-1 corneal infections cause long-term alterations of the corneal microenvironment that provide CD4-dependent innate resistance to subsequent infections by antigenically unrelated pathogens.
Flow Cytometry
del Rio, M. L., et al. (2012). "Selective blockade of herpesvirus entry mediator-B and T lymphocyte attenuator pathway ameliorates acute graft-versus-host reaction" J Immunol 188(10): 4885-4896. PubMed
The cosignaling network mediated by the herpesvirus entry mediator (HVEM; TNFRSF14) functions as a dual directional system that involves proinflammatory ligand, lymphotoxin that exhibits inducible expression and competes with HSV glycoprotein D for HVEM, a receptor expressed by T lymphocytes (LIGHT; TNFSF14), and the inhibitory Ig family member B and T lymphocyte attenuator (BTLA). To dissect the differential contributions of HVEM/BTLA and HVEM/LIGHT interactions, topographically-specific, competitive, and nonblocking anti-HVEM Abs that inhibit BTLA binding, but not LIGHT, were developed. We demonstrate that a BTLA-specific competitor attenuated the course of acute graft-versus-host reaction in a murine F(1) transfer semiallogeneic model. Selective HVEM/BTLA blockade did not inhibit donor T cell infiltration into graft-versus-host reaction target organs, but decreased the functional activity of the alloreactive T cells. These results highlight the critical role of HVEM/BTLA pathway in the control of the allogeneic immune response and identify a new therapeutic target for transplantation and autoimmune diseases.
Flow Cytometry
Lubick, K., et al. (2007). "Securinine, a GABAA receptor antagonist, enhances macrophage clearance of phase II C. burnetii: comparison with TLR agonists" J Leukoc Biol 82(5): 1062-1069. PubMed
Innate immune cell stimulation represents a complementary approach to vaccines and antimicrobial drugs to counter infectious disease. We have used assays of macrophage activation and in vitro and in vivo phase II Coxiella burnetii infection models to compare and contrast the activity of a novel innate immune cell agonist, securinine, with known TLR agonists. As expected, TLR agonists, such as LPS (TLR4) and fibroblast-stimulating lipopeptide-1 (FSL-1; TLR2), induced macrophage activation and increased macrophage killing of phase II C. burnetii in vitro. FSL-1 also induced accelerated killing of C. burnetii in vivo. Securinine, a gamma-aminobutyric acid type A receptor antagonist, was found to induce TLR-independent macrophage activation in vitro, leading to IL-8 secretion, L-selectin down-regulation, and CD11b and MHC Class II antigen up-regulation. As seen with the TLR agonists, securinine also induced accelerated macrophage killing of C. burnetii in vitro and in vivo. In summary, as predicted by the literature, TLR agonists enhance macrophage killing of phase II C. burnetii in vitro, and at least for TLR2 agonists, this activity occurs in vivo as well. Securinine represents a novel macrophage agonist, which has similar effects as TLR agonists in this model yet apparently, does not act through known TLRs. Securinine has minimal toxicity in vivo, suggesting it or structurally similar compounds may represent novel, therapeutic adjuvants, which increase resistance to intracellular pathogens.
Flow Cytometry
Duraiswamy, N., et al. (1994). "Distinction of class II MHC+ Langerhans cell-like interstitial dendritic antigen-presenting cells in murine dermis from dermal macrophages" J Invest Dermatol 103(5): 678-683. PubMed
Dermal cells are capable of initiating contact-hypersensitivity responses but the precise identification of the antigen-presenting cell within murine dermis is lacking. Class II major histocompatibility complex (MHC)+ cells with dendritic shape and lacking endothelial factor VIII but expressing the dendritic antigen-presenting cell marker NLDC-145 were observed in the perivascular and interstitial dermis of BALB/c and C3H/HeN skin. The heterogeneous class II MHC+ cells could be divided into two subsets: each was class II MHC+ CD45+ (bone marrow derived) GR-1- (non-neutrophil/macrophage) CD3- (non T), but one subset was CD11b+ (beta 2 integrin) and the other was CD11b-. Ultrastructural examination of class II MHC+ cells revealed the presence of a Langerhans cell-like/indeterminant cell subset with indented nuclei, dendritic morphology, active cytoplasm, and dense intermediate filaments. Phagolysomes and Birbeck granules were not observed in such cells, indicating these were distinct from dermal macrophages and from classical epidermal Langerhans cells, respectively. Cells with a monocyte/macrophage ultrastructural appearance were also noted, likely representing the class II MHC subset expressing CD11b and Ly6c (monocyte/endothelial antigen). Dermal cells in suspension were capable of processing and presenting large protein antigens to antigen-specific T-cell hybridomas; dermal cells also induced the syngeneic mixed lymphocyte reaction. The dermal antigen-presentation activities were totally abrogated by removal of class II MHC+ cells, but not by removal of CD11b+ cells or Ly6c+ cells, indicating that potent antigen-presenting cell activity was restricted to the class II MHC+ CD11b- Ly6c- subset (Langerhans cell-like/indeterminant cells). In conclusion, within a complex array of dermal leukocytes a murine dermal class II MHC+ cell population expressing a Langerhans cell-like/dendritic antigen-presenting cell phenotype and exhibiting potent antigen processing and presenting activity can be identified. The positioning of potent interstitial dendritic antigen-presenting cells at the interface of the vasculature with the dermal interstitium provides rapid access to an antigen-presenting cell as T cells first egress into the skin.
Flow Cytometry
Jutila, M. A., et al. (1988). "Ly-6C is a monocyte/macrophage and endothelial cell differentiation antigen regulated by interferon-gamma" Eur J Immunol 18(11): 1819-1826. PubMed
Using a new Ly-6C-specific antibody (Monts-1) we show that this class of antigens are differentially expressed on monocytes/macrophages and endothelial cells. Recently elicited peritoneal exudate Mac-1+ mononuclear cells, as well as Mac-1+ mononuclear cells in the bone marrow and in the peripheral blood, express high levels of Ly-6C. Ly-6C+ mononuclear Mac-1+ cells are absent in normal uninflamed skin, but are present in high numbers in skin lesions 3 days after the s.c. injection of lipopolysaccharide, concanavalin A or complete Freund’s adjuvant. In addition, large Ly-6C+ mononuclear cells are predominant in chronic granulomas induced by complete Freund’s adjuvant. Resident macrophages in a variety of tissues express low levels or in many cases do not express Ly-6C. Two out of three monocyte-like cell lines are Ly-6C+, whereas macrophage-like cell lines are negative. Ly-6C+ monocytes/macrophages lose the Ly-6C antigen within 24 h after in vitro culture. Ly-6C- cultured monocytes and Ly-6C- monocyte-like cell lines, but not fully differentiated macrophages and macrophage-like cell lines, can be induced to express the Ly-6C antigen by interferon-gamma. A population of small vessel endothelial cells in diverse tissues also express high levels of Ly-6C. The present findings suggest that the Ly-6C antigen family, shown by others to be involved in T cell activation, may have more general importance in immune responses and cellular differentiation than previously appreciated.
Candida-induced granulocytic myeloid-derived suppressor cells are protective against polymicrobial sepsis.
In mBio on 31 October 2023 by Esher, S. K., Harriett, A. J., et al.
PubMed
Polymicrobial intra-abdominal infections are serious clinical infections that can lead to life-threatening sepsis, which is difficult to treat in part due to the complex and dynamic inflammatory responses involved. Our prior studies demonstrated that immunization with low-virulence Candida species can provide strong protection against lethal polymicrobial sepsis challenge in mice. This long-lived protection was found to be mediated by trained Gr-1+ polymorphonuclear leukocytes with features resembling myeloid-derived suppressor cells (MDSCs). Here we definitively characterize these cells as MDSCs and demonstrate that their mechanism of protection involves the abrogation of lethal inflammation, in part through the action of the anti-inflammatory cytokine interleukin (IL)-10. These studies highlight the role of MDSCs and IL-10 in controlling acute lethal inflammation and give support for the utility of trained tolerogenic immune responses in the clinical treatment of sepsis.
- Cancer Research,
- Immunology and Microbiology,
- Mus musculus (House mouse)
In situ tumour arrays reveal early environmental control of cancer immunity.
In Nature on 1 June 2023 by Ortiz-Muñoz, G., Brown, M., et al.
PubMed
The immune phenotype of a tumour is a key predictor of its response to immunotherapy1-4. Patients who respond to checkpoint blockade generally present with immune-inflamed5-7 tumours that are highly infiltrated by T cells. However, not all inflamed tumours respond to therapy, and even lower response rates occur among tumours that lack T cells (immune desert) or that spatially exclude T cells to the periphery of the tumour lesion (immune excluded)8. Despite the importance of these tumour immune phenotypes in patients, little is known about their development, heterogeneity or dynamics owing to the technical difficulty of tracking these features in situ. Here we introduce skin tumour array by microporation (STAMP)-a preclinical approach that combines high-throughput time-lapse imaging with next-generation sequencing of tumour arrays. Using STAMP, we followed the development of thousands of arrayed tumours in vivo to show that tumour immune phenotypes and outcomes vary between adjacent tumours and are controlled by local factors within the tumour microenvironment. Particularly, the recruitment of T cells by fibroblasts and monocytes into the tumour core was supportive of T cell cytotoxic activity and tumour rejection. Tumour immune phenotypes were dynamic over time and an early conversion to an immune-inflamed phenotype was predictive of spontaneous or therapy-induced tumour rejection. Thus, STAMP captures the dynamic relationships of the spatial, cellular and molecular components of tumour rejection and has the potential to translate therapeutic concepts into successful clinical strategies. © 2023. The Author(s).
- Mus musculus (House mouse),
- Immunology and Microbiology,
- Neuroscience
Temporal tracking of microglial and monocyte single-cell transcriptomics in lethal flavivirus infection.
In Acta Neuropathologica Communications on 4 April 2023 by Spiteri, A. G., Wishart, C. L., et al.
PubMed
As the resident parenchymal myeloid population in the central nervous system (CNS), microglia are strategically positioned to respond to neurotropic virus invasion and have been implicated in promoting both disease resolution and progression in the acute and post-infectious phase of virus encephalitis. In a mouse model of West Nile virus encephalitis (WNE), infection of the CNS results in recruitment of large numbers of peripheral immune cells into the brain, the majority being nitric oxide (NO)-producing Ly6Chi inflammatory monocyte-derived cells (MCs). In this model, these cells enhance immunopathology and mortality. However, the contribution of microglia to this response is currently undefined. Here we used a combination of experimental tools, including single-cell RNA sequencing (scRNA-seq), microglia and MC depletion reagents, high-dimensional spectral cytometry and computational algorithms to dissect the differential contribution of microglia and MCs to the anti-viral immune response in severe neuroinflammation seen in WNE. Intriguingly, analysis of scRNA-seq data revealed 6 unique microglia and 3 unique MC clusters that were predominantly timepoint-specific, demonstrating substantial transcriptional adaptation with disease progression over the course of WNE. While microglia and MC adopted unique gene expression profiles, gene ontology enrichment analysis, coupled with microglia and MC depletion studies, demonstrated a role for both of these cells in the trafficking of peripheral immune cells into the CNS, T cell responses and viral clearance. Over the course of infection, microglia transitioned from a homeostatic to an anti-viral and then into an immune cell-recruiting phenotype. Conversely, MC adopted antigen-presenting, immune cell-recruiting and NO-producing phenotypes, which all had anti-viral function. Overall, this study defines for the first time the single-cell transcriptomic responses of microglia and MCs over the course of WNE, demonstrating both protective and pathological roles of these cells that could potentially be targeted for differential therapeutic intervention to dampen immune-mediated pathology, while maintaining viral clearance functions. © 2023. The Author(s).
- Mus musculus (House mouse),
- Immunology and Microbiology
PLX5622 Reduces Disease Severity in Lethal CNS Infection by Off-Target Inhibition of Peripheral Inflammatory Monocyte Production.
In Frontiers in Immunology on 12 April 2022 by Spiteri, A. G., Ni, D., et al.
PubMed
PLX5622 is a CSF-1R inhibitor and microglia-depleting reagent, widely used to investigate the biology of this central nervous system (CNS)-resident myeloid population, but the indirect or off-target effects of this agent remain largely unexplored. In a murine model of severe neuroinflammation induced by West Nile virus encephalitis (WNE), we showed PLX5622 efficiently depleted both microglia and a sub-population of border-associated macrophages in the CNS. However, PLX5622 also significantly depleted mature Ly6Chi monocytes in the bone marrow (BM), inhibiting their proliferation and lethal recruitment into the infected brain, reducing neuroinflammation and clinical disease scores. Notably, in addition, BM dendritic cell subsets, plasmacytoid DC and classical DC, were depleted differentially in infected and uninfected mice. Confirming its protective effect in WNE, cessation of PLX5622 treatment exacerbated disease scores and was associated with robust repopulation of microglia, rebound BM monopoiesis and markedly increased inflammatory monocyte infiltration into the CNS. Monoclonal anti-CSF-1R antibody blockade late in WNE also impeded BM monocyte proliferation and recruitment to the brain, suggesting that the protective effect of PLX5622 is via the inhibition of CSF-1R, rather than other kinase targets. Importantly, BrdU incorporation in PLX5622-treated mice, suggest remaining microglia proliferate independently of CSF-1 in WNE. Our study uncovers significantly broader effects of PLX5622 on the myeloid lineage beyond microglia depletion, advising caution in the interpretation of PLX5622 data as microglia-specific. However, this work also strikingly demonstrates the unexpected therapeutic potential of this molecule in CNS viral infection, as well as other monocyte-mediated diseases. Copyright © 2022 Spiteri, Ni, Ling, Macia, Campbell, Hofer and King.
- In Vivo,
- Homo sapiens (Human),
- Cancer Research,
- Immunology and Microbiology
Modulating tumor infiltrating myeloid cells to enhance bispecific antibody-driven T cell infiltration and anti-tumor response.
In Journal of Hematology & Oncology on 8 September 2021 by Park, J. A., Wang, L., et al.
PubMed
Tumor microenvironment (TME) is a dynamic cellular milieu to promote tumor angiogenesis, growth, proliferation, and metastasis, while derailing the host anti-tumor response. TME impedes bispecific antibody (BsAb) or chimeric antigen receptor (CAR)-driven T cells infiltration, survival, and cytotoxic efficacy. Modulating tumor infiltrating myeloid cells (TIMs) could potentially improve the efficacy of BsAb. We evaluated the effects of TIM modulation on BsAb-driven T cell infiltration into tumors, their persistence, and in vivo anti-tumor response. Anti-GD2 BsAb and anti-HER2 BsAb built on IgG-[L]-scFv platform were tested against human cancer xenografts in BALB-Rag2-/-IL-2R-γc-KO (BRG) mice. Depleting antibodies specific for polymorphonuclear myeloid-derived suppressor cell (PMN-MDSC), monocytic MDSC (M-MDSC), and tumor associated macrophage (TAM) were used to study the role of each TIM component. Dexamethasone, an established anti-inflammatory agent, was tested for its effect on TIMs. BsAb-driven T cells recruited myeloid cells into human tumor xenografts. Each TIM targeting therapy depleted cells of interest in blood and in tumors. Depletion of PMN-MDSCs, M-MDSCs, and particularly TAMs was associated with enhanced T cell infiltration into tumors, significantly improving tumor control and survival in multiple cancer xenograft models. Dexamethasone premedication depleted monocytes in circulation and TAMs in tumors, enhanced BsAb-driven T cell infiltration, and anti-tumor response with survival benefit. Reducing TIMs markedly enhanced anti-tumor effects of BsAb-based T cell immunotherapy by improving intratumoral T cell infiltration and persistence. TAM depletion was more effective than PMN- or M-MDSCs depletion at boosting the anti-tumor response of T cell engaging BsAb. © 2021. The Author(s).
- Block,
- Mus musculus (House mouse),
- Immunology and Microbiology,
- Neuroscience
High-parameter cytometry unmasks microglial cell spatio-temporal response kinetics in severe neuroinflammatory disease.
In Journal of Neuroinflammation on 26 July 2021 by Spiteri, A. G., Terry, R. L., et al.
PubMed
Differentiating infiltrating myeloid cells from resident microglia in neuroinflammatory disease is challenging, because bone marrow-derived inflammatory monocytes infiltrating the inflamed brain adopt a 'microglia-like' phenotype. This precludes the accurate identification of either cell type without genetic manipulation, which is important to understand their temporal contribution to disease and inform effective intervention in its pathogenesis. During West Nile virus (WNV) encephalitis, widespread neuronal infection drives substantial CNS infiltration of inflammatory monocytes, causing severe immunopathology and/or death, but the role of microglia in this remains unclear. Using high-parameter cytometry and dimensionality-reduction, we devised a simple, novel gating strategy to identify microglia and infiltrating myeloid cells during WNV-infection. Validating our strategy, we (1) blocked the entry of infiltrating myeloid populations from peripheral blood using monoclonal blocking antibodies, (2) adoptively transferred BM-derived monocytes and tracked their phenotypic changes after infiltration and (3) labelled peripheral leukocytes that infiltrate into the brain with an intravenous dye. We demonstrated that myeloid immigrants populated only the identified macrophage gates, while PLX5622 depletion reduced all 4 subsets defined by the microglial gates. Using this gating approach, we identified four consistent microglia subsets in the homeostatic and WNV-infected brain. These were P2RY12hi CD86-, P2RY12hi CD86+ and P2RY12lo CD86- P2RY12lo CD86+. During infection, 2 further populations were identified as 'inflammatory' and 'microglia-like' macrophages, recruited from the bone marrow. Detailed kinetic analysis showed significant increases in the proportions of both P2RY12lo microglia subsets in all anatomical areas, largely at the expense of the P2RY12hi CD86- subset, with the latter undergoing compensatory proliferation, suggesting replenishment of, and differentiation from this subset in response to infection. Microglia altered their morphology early in infection, with all cells adopting temporal and regional disease-specific phenotypes. Late in disease, microglia produced IL-12, downregulated CX3CR1, F4/80 and TMEM119 and underwent apoptosis. Infiltrating macrophages expressed both TMEM119 and P2RY12 de novo, with the microglia-like subset notably exhibiting the highest proportional myeloid population death. Our approach enables detailed kinetic analysis of resident vs infiltrating myeloid cells in a wide range of neuroinflammatory models without non-physiological manipulation. This will more clearly inform potential therapeutic approaches that specifically modulate these cells. © 2021. The Author(s).
- Mus musculus (House mouse),
- Neuroscience
High-parameter Cytometry Unmasks Microglial Cell Spatio-temporal Response Kinetics in Severe Neuroinflammatory Disease.
Preprint on Research Square on 7 April 2021 by Spiteri, A. G., Terry, R. L., et al.
PubMed
h4>Background: /h4> Differentiating infiltrating myeloid cells from resident microglia in neuroinflammatory disease is challenging, because bone marrow-derived inflammatory monocytes infiltrating the inflamed brain adopt a ‘microglia-like’ phenotype. This precludes the accurate identification of either cell type without genetic manipulation, which is important to understand their temporal contribution to disease and inform effective intervention in its pathogenesis. During West Nile virus (WNV) encephalitis, widespread neuronal infection drives substantial CNS infiltration of inflammatory monocytes, causing severe immunopathology and/or death, but the role of microglia in this remains unclear. h4>Methods: /h4>: Using high-parameter cytometry and dimensionality-reduction, we devised a simple, novel gating strategy to identify microglia and infiltrating myeloid cells under extreme inflammatory conditions. Validating our strategy we 1) blocked the entry of infiltrating myeloid populations from peripheral blood using monoclonal blocking antibodies, 2) adoptively transferred BM-derived monocytes and tracked their phenotypic changes after infiltration and 3) labelled peripheral leukocytes that infiltrate into the brain with an intravenous dye. We demonstrated that myeloid immigrants populated only the identified macrophage gates, while PLX5622 depletion reduced all 4 subsets defined by the microglial gates. h4>Results: /h4>: Using this novel gating approach, we identified four consistent microglia subsets in the homeostatic and WNV-infected brain. These were P2RY12 hi CD86 - , P2RY12 hi CD86 + , and P2RY12 lo CD86 - P2RY12 lo CD86 + . During infection, 2 further populations were identified as inflammatory and microglia-like macrophages, recruited from the bone marrow. Detailed kinetic analysis showed significant increases in the proportions of both P2RY12 lo microglia subsets in all anatomical areas, largely at the expense of the P2RY12 hi CD86 - subset, with the latter undergoing compensatory proliferation, suggesting replenishment of, and differentiation from this subset in response to infection. Microglia altered their morphology early in infection, with all cells adopting temporal and regional disease-specific phenotypes. Late in disease, microglia produced IL-12, downregulated CX3CR1, F4/80 and TMEM119 and underwent apoptosis. Infiltrating macrophages expressed both TMEM119 and P2RY12 de novo, with the microglia-like subset notably exhibiting the highest proportional myeloid population death. h4>Conclusions: /h4>: Our approach enables detailed kinetic analysis of resident vs infiltrating myeloid cells in a wide range of neuroinflammatory models without non-physiological manipulation. This will more clearly inform potential therapeutic approaches that specifically modulate these cells.
- Cancer Research
The association between monocytic myeloid-derived suppressor cells levels and the anti-tumor efficacy of anti-PD-1 therapy in NSCLC patients.
In Translational Oncology on 1 December 2020 by Feng, J., Chen, S., et al.
PubMed
Monocytic myeloid-derived suppressor cells (M-MDSCs), granulocytic MDSC (G-MDSCs) and regulatory T cells (Tregs) inhibit adaptive anti-tumor immunity and undermine the efficacy of anti-PD-1 therapy. However, the impact of anti-PD-1 treatment on these immunosuppressive cells has not been clearly defined in non-small cell lung cancer (NSCLC). In this retrospective study, 27 advanced NSCLC patients were divided into partial response (PR), stable disease (SD), and progressive disease (PD) groups. The impact of anti-PD-1 therapy on circulating Tregs, G-MDSCs, and M-MDSCs was assessed by flow cytometer. Here, we found that anti-PD-1 treatment boosted circulating Tregs levels, which presented the most remarkable augment during the first two therapeutic cycles, in NSCLC patients. In contrast, anti-PD-1 therapy did not overall change G-MDSCs and M-MDSCs levels. However, the PR group had a higher baseline level of M-MDSCs, which exhibited a significant decrease after the first cycle of anti-PD-1 treatment. Besides, M-MDSCs levels in the PR group were maintained at a low level in the following therapeutic cycles. Consistently, Tregs levels robustly increased in the syngeneic tumor model after anti-mouse PD-1 Ab treatment. Accordingly, M-MDSCs neutralization by anti-mouse ly6c Ab enhanced the anti-tumor efficacy of anti-PD-1 therapy in mice. Finally, the decreased M-MDSCs levels were associated with the enhanced effector CD8+ T cells expansion in the PR group and mice. In conclusion, anti-PD-1 therapy upregulates Tregs levels in NSCLC patients, and the M-MDSC levels are associated with the anti-tumor efficacy of anti-PD-1 treatment. Neutralization of M-MDSCs may be a promising option to augment anti-PD-1 therapy efficacy in NSCLC. Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
- Cancer Research,
- Immunology and Microbiology
TET2 promotes anti-tumor immunity by governing G-MDSCs and CD8+ T-cell numbers.
In EMBO Reports on 5 October 2020 by Li, S., Feng, J., et al.
PubMed
The host immune response is a fundamental mechanism for attenuating cancer progression. Here we report a role for the DNA demethylase and tumor suppressor TET2 in host anti-tumor immunity. Deletion of Tet2 in mice elevates IL-6 levels upon tumor challenge. Elevated IL-6 stimulates immunosuppressive granulocytic myeloid-derived suppressor cells (G-MDSCs), which in turn reduce CD8+ T cells upon tumor challenge. Consequently, systematic knockout of Tet2 in mice leads to accelerated syngeneic tumor growth, which is constrained by anti-PD-1 blockade. Removal of G-MDSCs by the anti-mouse Ly6g antibodies restores CD8+ T-cell numbers in Tet2-/- mice and reboots their anti-tumor activity. Importantly, anti-IL-6 antibody treatment blocks the expansion of G-MDSCs and inhibits syngeneic tumor growth. Collectively, these findings reveal a TET2-mediated IL-6/G-MDSCs/CD8+ T-cell immune response cascade that safeguards host adaptive anti-tumor immunity, offering a cell non-autonomous mechanism of TET2 for tumor suppression. © 2020 The Authors.
- Mus musculus (House mouse),
- Cancer Research
Myeloid-Derived Suppressor Cell Subsets Drive Glioblastoma Growth in a Sex-Specific Manner.
In Cancer Discovery on 1 August 2020 by Bayik, D., Zhou, Y., et al.
PubMed
Myeloid-derived suppressor cells (MDSC) that block antitumor immunity are elevated in glioblastoma (GBM) patient blood and tumors. However, the distinct contributions of monocytic (mMDSC) versus granulocytic (gMDSC) subsets have yet to be determined. In mouse models of GBM, we observed that mMDSCs were enriched in the male tumors, whereas gMDSCs were elevated in the blood of females. Depletion of gMDSCs extended survival only in female mice. Using gene-expression signatures coupled with network medicine analysis, we demonstrated in preclinical models that mMDSCs could be targeted with antiproliferative agents in males, whereas gMDSC function could be inhibited by IL1β blockade in females. Analysis of patient data confirmed that proliferating mMDSCs were predominant in male tumors and that a high gMDSC/IL1β gene signature correlated with poor prognosis in female patients. These findings demonstrate that MDSC subsets differentially drive immune suppression in a sex-specific manner and can be leveraged for therapeutic intervention in GBM. SIGNIFICANCE: Sexual dimorphism at the level of MDSC subset prevalence, localization, and gene-expression profile constitutes a therapeutic opportunity. Our results indicate that chemotherapy can be used to target mMDSCs in males, whereas IL1 pathway inhibitors can provide benefit to females via inhibition of gMDSCs.See related commentary by Gabrilovich et al., p. 1100.This article is highlighted in the In This Issue feature, p. 1079. ©2020 American Association for Cancer Research.
- Cancer Research
Type I IFN, Ly6C+ cells, and Phagocytes Support Suppression of Peritoneal Carcinomatosis Elicited by a TLR and CLR Agonist Combination.
In Molecular Cancer Therapeutics on 1 June 2020 by Dyevoich, A. M. & Haas, K. M.
PubMed
Metastatic cancer involving spread to the peritoneal cavity is referred to as peritoneal carcinomatosis and has a very poor prognosis. Our previous study demonstrated a Toll-like receptor and C-type lectin receptor agonist pairing of monophosphoryl lipid A (MPL) and trehalose-6,6'-dicorynomycolate (TDCM) effectively inhibits tumor growth and ascites development following TA3-Ha and EL4 challenge through a mechanism dependent on B-1a cell-produced natural IgM and complement. In this study, we investigated additional players in the MPL/TDCM-elicited response. MPL/TDCM treatment rapidly increased type I IFN levels in the peritoneal cavity along with myeloid cell numbers, including macrophages and Ly6Chi monocytes. Type I IFN receptor (IFNAR1-/-) mice produced tumor-reactive IgM following MPL/TDCM treatment, but failed to recruit Ly6C+ monocytes and were not afforded protection during tumor challenges. Clodronate liposome depletion of phagocytic cells, as well as targeted depletion of Ly6C+ cells, also ablated MPL/TDCM-induced protection. Cytotoxic mediators known to be produced by these cells were required for effects. TNFα was required for effective TA3-Ha killing and nitric oxide was required for EL4 killing. Collectively, these data reveal a model whereby MPL/TDCM-elicited antitumor effects strongly depend on innate cell responses, with B-1a cell-produced tumor-reactive IgM and complement pairing with myeloid cell-produced cytotoxic mediators to effectively eradicate tumors in the peritoneal cavity. ©2020 American Association for Cancer Research.
- FC/FACS,
- Homo sapiens (Human),
- Cancer Research,
- Pathology
Myeloid-Derived Lymphatic Endothelial Cell Progenitors Significantly Contribute to Lymphatic Metastasis in Clinical Breast Cancer.
In The American Journal of Pathology on 1 November 2019 by Volk-Draper, L., Patel, R., et al.
PubMed
Lymphatic metastasis is a high-impact prognostic factor for mortality of breast cancer (BC) patients, and it directly depends on tumor-associated lymphatic vessels. We previously reported that lipopolysaccharide-induced inflammatory lymphangiogenesis is strongly promoted by myeloid-derived lymphatic endothelial cell progenitors (M-LECPs) derived from the bone marrow (BM). As BC recruits massive numbers of provascular myeloid cells, we hypothesized that M-LECPs, within this recruited population, are specifically programmed to promote tumor lymphatics that increase lymph node metastasis. In support of this hypothesis, high levels of M-LECPs were found in peripheral blood and tumor tissues of BC patients. Moreover, the density of M-LECPs and lymphatic vessels positive for myeloid marker proteins strongly correlated with patient node status. It was also established that tumor M-LECPs coexpress lymphatic-specific, stem/progenitor and M2-type macrophage markers that indicate their BM hematopoietic-myeloid origin and distinguish them from mature lymphatic endothelial cells, tumor-infiltrating lymphoid cells, and tissue-resident macrophages. Using four orthotopic BC models, we show that mouse M-LECPs are similarly recruited to tumors and integrate into preexisting lymphatics. Finally, we demonstrate that adoptive transfer of in vitro differentiated M-LECPs, but not naïve or nondifferentiated BM cells, significantly increased metastatic burden in ipsilateral lymph nodes. These data support a causative role of BC-induced lymphatic progenitors in tumor lymphangiogenesis and suggest molecular targets for their inhibition. Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
- Mus musculus (House mouse),
- Genetics
Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes.
In Nature Communications on 29 October 2018 by Veiga, N., Goldsmith, M., et al.
PubMed
Therapeutic alteration of gene expression in vivo can be achieved by delivering nucleic acids (e.g., mRNA, siRNA) using nanoparticles. Recent progress in modified messenger RNA (mmRNA) synthesis facilitated the development of lipid nanoparticles (LNPs) loaded with mmRNA as a promising tool for in vivo protein expression. Although progress have been made with mmRNA-LNPs based protein expression in hepatocytes, cell specificity is still a major challenge. Moreover, selective protein expression is essential for an improved therapeutic effect, due to the heterogeneous nature of diseases. Here, we present a precision protein expression strategy in Ly6c+ inflammatory leukocytes in inflammatory bowel disease (IBD) induced mice. We demonstrate a therapeutic effect in an IBD model by targeted expression of the interleukin 10 in Ly6c+ inflammatory leukocytes. A selective mmRNA expression strategy has tremendous therapeutic potential in IBD and can ultimately become a novel therapeutic modality in many other diseases.
- Immunology and Microbiology
Inflammatory Ly6Chigh Monocytes Protect against Candidiasis through IL-15-Driven NK Cell/Neutrophil Activation.
In Immunity on 20 June 2017 by Domínguez-Andrés, J., Feo-Lucas, L., et al.
PubMed
Neutrophils play a crucial role in defense against systemic candidiasis, a disease associated with a high mortality rate in patients receiving immunosuppressive therapy, although the early immune mechanisms that boost the candidacidal activity of neutrophils remain to be defined in depth. Here, we used a murine model of systemic candidiasis to explore the role of inflammatory Ly6Chigh monocytes in NK cell-mediated neutrophil activation during the innate immune response against C. albicans. We found that efficient anti-Candida immunity required a collaborative response between the spleen and kidney, which relied on type I interferon-dependent IL-15 production by spleen inflammatory Ly6Chigh monocytes to drive efficient activation and GM-CSF release by spleen NK cells; this in turn was necessary to boost the Candida killing potential of kidney neutrophils. Our findings unveil a role for IL-15 as a critical mediator in defense against systemic candidiasis and hold promise for the design of IL-15-based antifungal immunotherapies. Copyright © 2017 Elsevier Inc. All rights reserved.
- Immunology and Microbiology
Subclinical Herpes Simplex Virus Type 1 Infections Provide Site-Specific Resistance to an Unrelated Pathogen.
In The Journal of Immunology on 15 February 2017 by Rowe, A. M., Yun, H., et al.
PubMed
HSV-1 infections of the cornea range in severity from minor transient discomfort to the blinding disease herpes stromal keratitis, yet most patients experience a single episode of epithelial keratitis followed by re-establishment of a clear cornea. We asked whether a single transient episode of HSV-1 epithelial keratitis causes long-term changes in the corneal microenvironment that influence immune responses to subsequent corneal infection or trauma. We showed that C57BL/6 mouse corneas infected with HSV-1 KOS, which induces transient herpes epithelial keratitis without herpes stromal keratitis sequelae, possessed a significant leukocytic infiltrate composed primarily of CD4+ T cells and macrophages along with elevated chemokines and cytokines that persisted without loss of corneal clarity (subclinical inflammation). Chemokine and cytokine expression was CD4+ T cell dependent, in that their production was significantly reduced by systemic CD4+ T cell depletion starting before infection, although short-term (3-d) local CD4+ T cell depletion postinfection did not influence chemokine levels in cornea. Corneas with subclinical inflammation developed significantly greater trauma-induced inflammation when they were recipients of syngeneic corneal transplants but also exhibited significantly increased resistance to infections by unrelated pathogens, such as pseudorabies virus. The resistance to pseudorabies virus was CD4+ T cell dependent, because it was eliminated by local CD4+ T cell depletion from the cornea. We conclude that transient HSV-1 corneal infections cause long-term alterations of the corneal microenvironment that provide CD4-dependent innate resistance to subsequent infections by antigenically unrelated pathogens. Copyright © 2017 by The American Association of Immunologists, Inc.